CitraGen is currently developing several Over-the-counter (OTC) drug products, which are under various stages of development and commercial launch. Some of our OTC drug products are First-to-Market, which will be marketed as Branded Consumer Health care products. These branded OTC drug products are scheduled for commercial launch starting from 4Q . We have filed two trademarks with USPTO for the branding of our specialty OTC products and First-to-market OTC products.
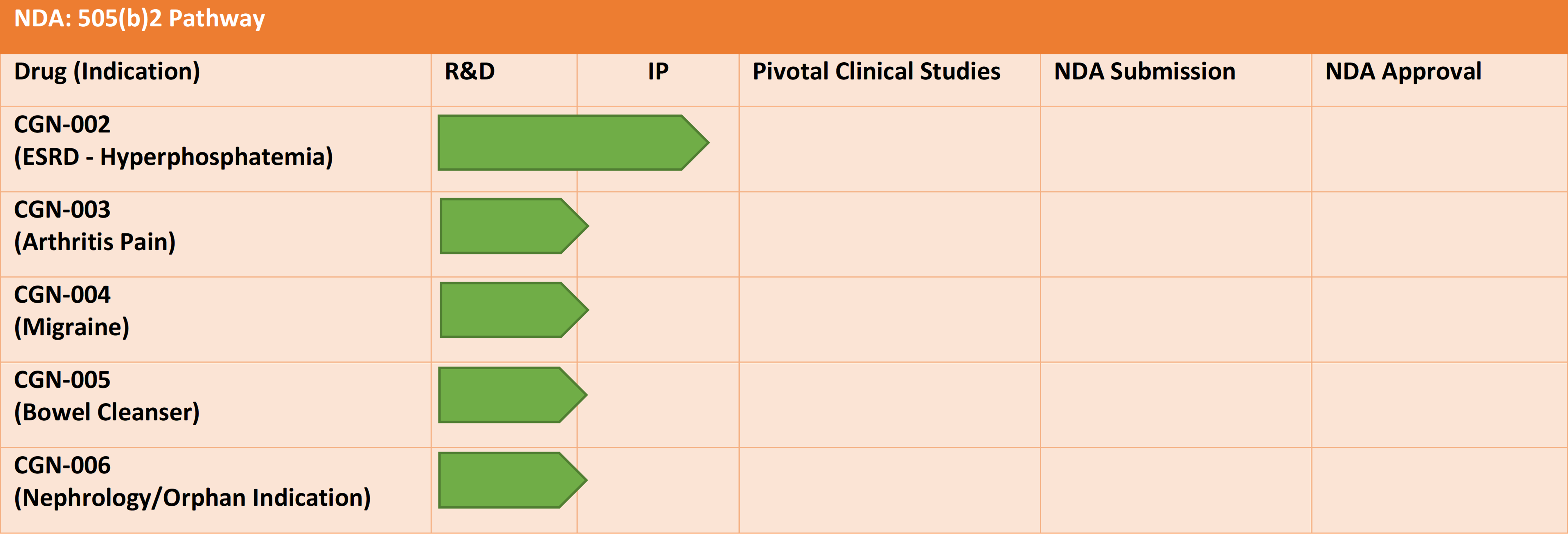
We are also currently developing the following lead NDA products (under the 505(b)2 regulatory pathway). These NDA products have an addressable market of over US$15 billion, globally.